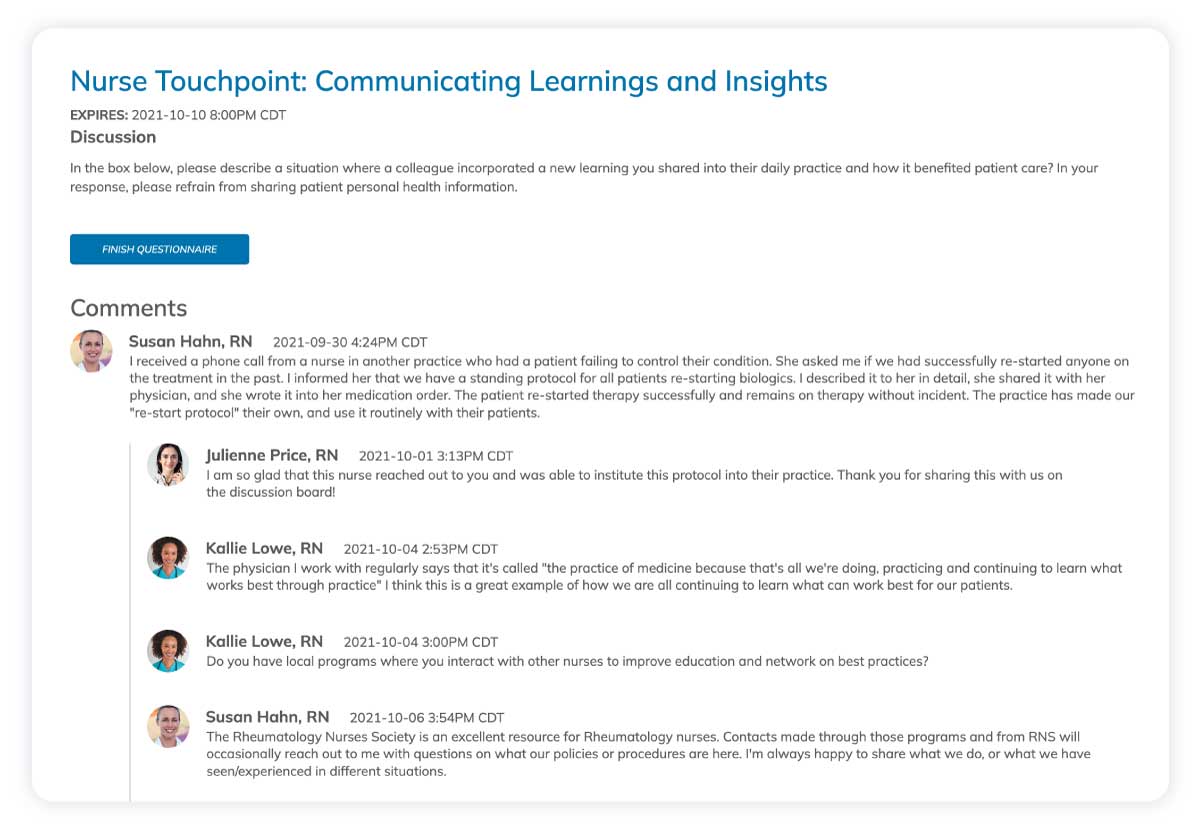
Sustained site motivation
Regular touchpoints and recognition help maintain site enthusiasm throughout lengthy trials. Sites feel valued and connected to the study's broader mission.
Enhanced data quality
Engaged sites produce better-quality data. Our platform helps maintain protocol compliance and thorough documentation through ongoing support and education.
Improved patient retention
Better-supported sites are better equipped to keep patients engaged. Shared best practices and resources help sites overcome common retention challenges.
Real-time problem solving
Quick identification and resolution of site challenges helps prevent small issues from becoming major problems. Our platform facilitates rapid communication and collaborative solution development.
Flexible integration
Our solution works seamlessly alongside your existing trial infrastructure. It’s compatible with CRO partnerships, integrated with patient data collection apps and supports recruitment programs.
Continuous collaboration
Many sponsors return for subsequent trial phases or new studies, typically reengaging after brief breaks between projects. Our platform maintains site relationships and institutional knowledge between studies.